Chhattisgarh: CBI to probe alleged irregularities in PSC Exam-2021
Chief Minister Vishnu Deo Sai during a Cabinet meeting on January 3 this year had decided to refer the matter to the CBI for a comprehensive inquiry.
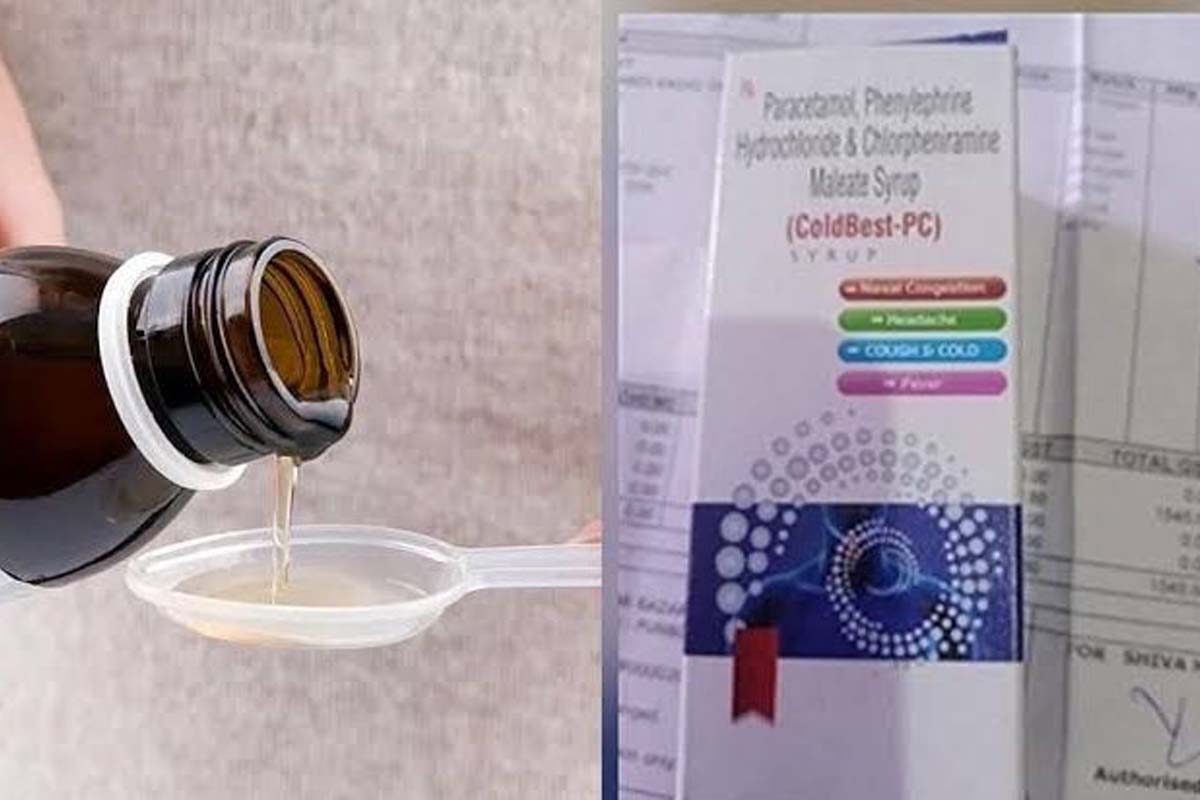
Mankotia said that the drug manufacturer had supplied the adulterated cough syrup in J&K and other states and this required a thorough probe by the CBI as similar fatalities elsewhere might have gone unreported.
[Representational Image]
Balwant Singh Mankotia, president of the Panthers Party and a former MLA of Udhampur, on Wednesday, demanded CBI enquiry into the death of 12 children after consuming a dose of “adulterated” cough syrup that was supplied by a Himachal Pradesh based drug manufacturer.
Mankotia said that the drug manufacturer had supplied the adulterated cough syrup in J&K and other states and this required a thorough probe by the CBI as similar fatalities elsewhere might have gone unreported.
These children suffering from cold in the Ramnagar area of Udhampur district were given the syrup in early January after which their health deteriorated and they died after renal failure.
Advertisement
Even after two months of the tragic incident, the authorities in J&K were mum on the serious issue and have so far failed to initiate legal or police action against the drug manufacturer. The union health ministry had sent a team of doctors when these deaths were initially suspected to be some epidemic.
Although authorities in Himachal Pradesh have now filed a case against the Amb based Digital Vision pharma unit that according to a report of the Chandigarh based regional drug testing laboratory had mixed high level of adulterant Diethylene Glycol that caused death of children, but this is not the first time that the drugs manufactured by the unit have been found sub-standard. Batches of seven drugs manufactured by the pharma unit between 2015 and 2020 were found sub-standard, according to a report.
A public health activist, Dinesh S. Thakur has expressed concern on the issue and in a series of tweets he questioned what action had the authorities taken against those manufacturing and supplying sub-standard drugs.
Advertisement