AstraZeneca gets approval to import breast cancer treatment drug in India
The India approval is based on a global, head-to-head, randomised, open-label, registrational Phase III trial of DESTINY Breast 03.
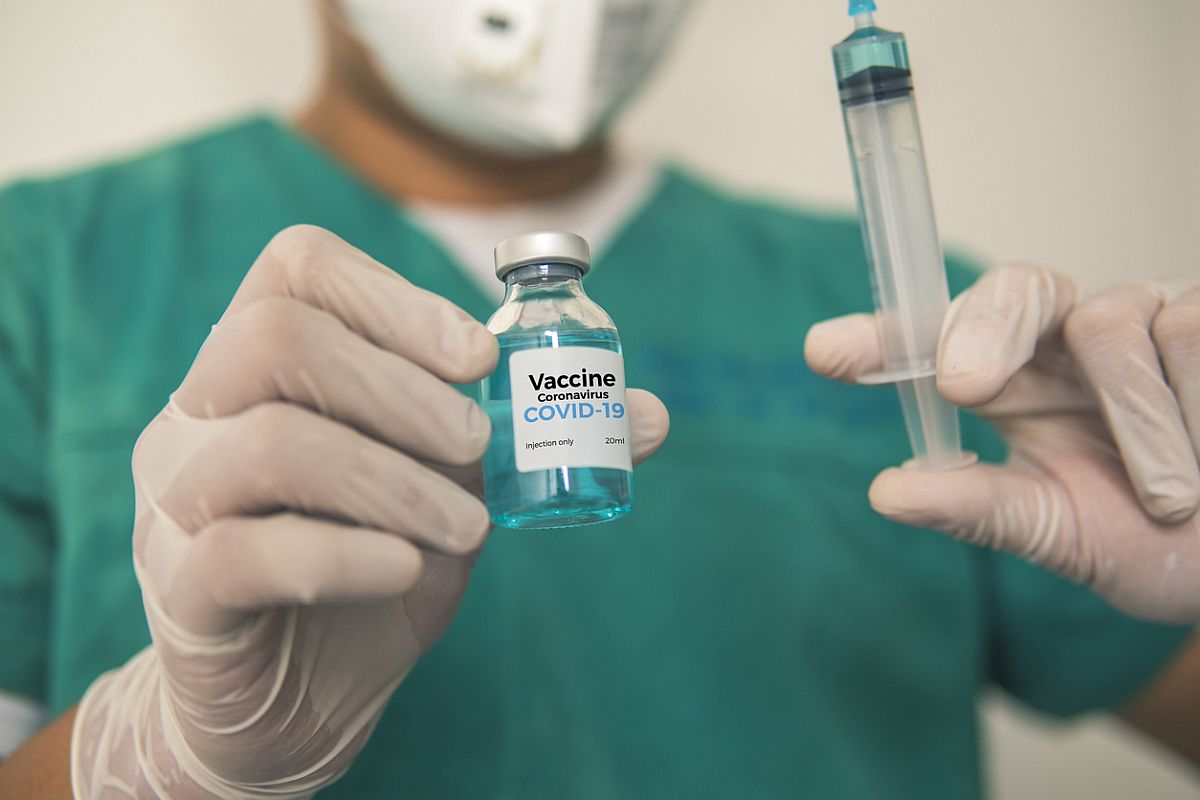
Oxford Institute vaccine Covishield which is being developed by Pune based Serum Institute and Bharat Biotech’s Covaxin has been approved by Drug Controller General of India (DCGI) on Sunday for emergency use for immunisation against Covid-19.
(Photo: iStock)
Oxford Institute vaccine Covishield which is being developed by Pune based Serum Institute and Bharat Biotech’s Covaxin has been approved by Drug Controller General of India (DCGI) on Sunday for emergency use for immunisation against Covid-19.
DCGI VG Somani said, “We’ll never approve anything if there is slightest of safety concern. The vaccines are 100 per cent safe,” adding that Oxford vaccine Covishield was found to be 70.42 per cent effective and Bharat Biotech’s Covaxin was found to be ‘safe and provides a robust immune response.’
Covishield was recommended for emergency use on January 1 and Covaxin was recommended for restricted use on January 2. Both the vaccines have to be administered in two doses and to be stored at temperatures between 2 and 8 degree celsius.
Advertisement
It would make every Indian proud that the two vaccines that have been given emergency use approval are made in India! This shows the eagerness of our scientific community to fulfil the dream of an Aatmanirbhar Bharat, at the root of which is care and compassion.
— Narendra Modi (@narendramodi) January 3, 2021
Soon after drug controller gave nod to both the vaccines, Prime Minister Narendra Modi tweeted, “It would make every Indian proud that the two vaccines that have been given emergency use approval are made in India! This shows the eagerness of our scientific community to fulfil the dream of an Aatmanirbhar Bharat, at the root of which is care and compassion.”
The DCGI said that Serum Institute has submitted data of over 73,000 participants and it is conducting trials for Phase 2 and 3 on 1,600 participants in the country. The recommendation was made for restricted use and the trials will continue, the controller added. The Oxford vaccine is already in use abroad with pharma company AstraZenca.
Bharat Biotech’s Covaxin which conducting trails with Indian Council of Medical Research (ICMR) and Phase 1 and 2 trials were conducted in around 800 people. The Phase 3 trial in on and 22,500 of 25,800 participants have been vaccinated.
The expert committee set up by the government reviewed Bharat Biotech’s data on ‘safety and immunogenicity’ and permitted it for ‘restricted use in emergency situation in public interest.’
Seem Institute’s chief Adar Poonawalla tweeted, “Happy new year, everyone! All the risks @SerumInstIndia took with stockpiling the vaccine, have finally paid off. COVISHIELD, India’s first COVID-19 vaccine is approved, safe, effective and ready to roll-out in the coming weeks.”
Happy new year, everyone! All the risks @SerumInstIndia took with stockpiling the vaccine, have finally paid off. COVISHIELD, India's first COVID-19 vaccine is approved, safe, effective and ready to roll-out in the coming weeks. pic.twitter.com/TcKh4bZIKK
— Adar Poonawalla (@adarpoonawalla) January 3, 2021
Union Health Minister Harsh Vardhan said, “A watershed moment in India’s famed battle against #COVID19 under leadership of PM Modi. Our wait for the vaccine is over with Covishield from Serum Institue of India and Covaxin from Bharat Biotech approved for emergency use in India.”
A watershed moment in India’s famed battle against #COVID19 under the charismatic leadership of Hon’ble PM Sh @narendramodi Ji !
Our wait for #COVID19vaccine is over with COVISHIELD from @SerumInstIndia & COVAXIN from @BharatBiotech approved for emergency use in India@PMOIndia pic.twitter.com/sqjsetqHnU
— Dr Harsh Vardhan (@drharshvardhan) January 3, 2021
On Saturday, selected hospitals across the country conducted the dry run for the vaccine. It was done to understand the best way to administer the vaccine and to identify the loopholes in the system. Union health Minister Harsh Vardhan said while reviewing the dry run on Saturday said that the vaccine would be given for free to the one crore healthcare workers and two crore frontline workers.
India in the last 24 hours has reported 18,177 cases and teetotal caseload stands at 1,03,23,965
Advertisement