Seminar discusses agriskills enhancement
The Department of Agriculture at Brainware University hosted a one-day National seminar on 20 April.
A gentler method of putting nitrogen into the soil is on its way.

Representational Image.
With world population and pressure on land set to increase, the demands on farm productivity, and hence, the use of chemical fertilisers, will be greater than before. The current means of producing chemical fertilisers, however, uses huge energy and causes huge carbon dioxide emission. The process infamously consumes three per cent of the world’s production of natural gas and releases three per cent of the world’s carbon emissions.
It is in this context, that Jun Wang, Liang Yu, Lin Hu, Gang Chen, Hongliang Xin and Xiaofeng Feng, from the University of Central Florida and the Virginia Polytechnic Institute and State University, report in the journal, Nature Communications, a method of producing ammonia — the raw material for fertilisers — without the high temperature and pressure that the industrial process demands. This may hence be the answer to the world’s urgent need to grow more food and yet contain carbon emissions.
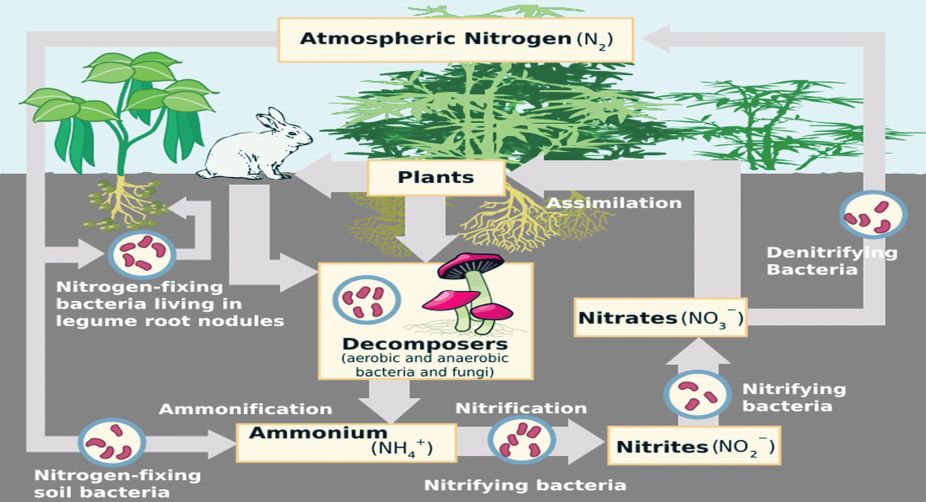
Nitrogen is a crucial element in the formation of vegetable tissue. Agriculture hence depends on sufficient nitrogen, in a form that can be used, being there in the soil. During aggressive cultivation, the nitrogen gets consumed faster than it can be restored by natural processes and the land loses fertility.
Advertisement
This limiting factor was overcome when ways to manufacture chemicals that pump nitrogen into the soil were found and since then, the production of grain and the production of fertliser have boomed.
As nitrogen, at 78 percent, is the most abundant gas in the atmosphere, one would expect that getting it into the soil should be routine. But this is not so as the nitrogen in the air, which is the end product of the nitrogen cycle, is not in the form that can be used.
Atmospheric nitrogen consists of molecules where two atoms of nitrogen are very firmly bound together. The strength of this bond does not allow nitrogen atoms to participate in reactions with other compounds. Before they can be used by processes of plant growth, the bond between the atoms needs to be broken and the same presented in a less firmly attached form.
Farmers have traditionally used compost, which is decomposed plant matter, or animal refuse, to release “active” nitrogen and fertilise their fields. The ammonia molecule, or NH3, also has usable nitrogen, and ammonia salts can be added to the soil as powerful fertilisers.
Creating ammonia, or its salts from the nitrogen in the air, in the lab, particularly in quantity, however, was not feasible till the Haber process was developed in the early 20th century. In this process, intense heat and high pressure, with the help of “helper” agents, or catalysts, get nitrogen atoms to separate and take on three hydrogen atoms in place of their normal partner, to form ammonia. The process, however, is energy intensive.
The reaction, depicted as N2+3H2= 2NH3, in fact, is exothermic, or one that gives off heat. This would suggest that the process needs no addition of heat but may benefit from cooling. This, however, is not true because the nitrogen molecule does not separate as two atoms, which can separately combine with hydrogen, unless it is heated to a few thousand degrees centigrade.
This is also true to an extent of the hydrogen molecule. Temperature, in turn, impedes the exothermic reaction, which hence proceeds only under very high pressure. Fortunately, things can be made easier by catalysts, or special substances that provide half-way stops that enable the reaction go through in stages.
The main catalyst in the Haber process is finely divided iron, on the surface of which the molecules loosen up, so that the N and H atoms are able to come in contact. Even so, the temperature needs to be about 550°C and the pressure over 200 atmospheres. As the efficiency of the reaction is about 15 percent, there have to be several passes before sufficient ammonia is produced.
Hydrogen gas also needs to be produced, which calls for power and high temperatures too. The whole process is thus energy intensive, fossil-fuel dependent and polluting. However, as it is the best one known for making ammonia at a very large scale, it has been extensively used and agriculture that does not use chemical fertiliser can no longer be viable.
If it is thus essential to have high temperature for turning out ammonia, how is it that natural processes, like microbes in the soil, and some plants, are able to create active nitrogen simply at the ambient temperature? The way nature does it is with the help of enzymes, complex molecules, which, incidentally, contain iron.
These depend, not on the brute force method of high temperature, where nitrogen atoms bound in molecules are battered apart, but on the property of the iron atom to gently loosen nitrogen bonds, so that supply of just a little energy can set the atoms free, long enough for them to combine with atoms of other elements.
Discovery of a similar, low-temperature process for producing ammonia, using electricity from renewable sources, would be an eco-friendly route to fertilisers, the paper in Nature Communications says. As ammonia is also a good energy source and burns without pollution, such a process could be a method of storing and retrieving surplus electric power, the paper says.
The quest for such a method, of course, has been on for decades. The formation of NH3 from N2 and H2O is essentially climbing back to extract N and H from the stable states of N2 and H2O. We say “climbing back” because NH3 readily burns in oxygen to yield nitrogen and water and it is the reverse that needs energy.
The approach to low-temperature methods to bring this about has hence followed nature’s lead and a number of catalysts, based on ruthenium, platinum, gold, iron and nickel have been developed, the paper says.
These catalysts, however, have generally shown low activity. Another concern, the paper says, is that in a water medium, catalysts that promote N2 dissociation also promote generation of hydrogen gas. This leads to the second process taking place, and the efficiency, for production of NH3, is less than one percent. The need is hence for an agent that promotes N2 dissociation and suppresses hydrogen evolution.
The current study reports encouraging results on using nanoparticles of palladium with a supporting base of carbon black — denoted as Pd/C. When used in a selected medium that suppresses the hydrogen evolution reaction, the catalyst enabled a reasonable level of NH3 production, needing the application of low electrical energy and with efficiency of 8.2 percent.
Trials using similarly prepared particles of gold and platinum, in place of palladium, showed that these metals were also capable of catalysing the reaction, but with much lower efficiency and less than 10 per cent of the yield.
Enquiry into how palladium turns out to be a unique catalyst for this reaction leads to insight into the mechanism of the action. “Our findings open up an avenue to develop efficient electrocatalysts for not only the electro-reduction of N2 to NH3, but also other challenging electrocatalytic reactions for renewable energy conversions,” the paper says.
The writer can be contacted at response@simplescience.in
Advertisement